Contact us:
Tel : +1(720)525-8971
Email: info@energesoft.com
2383 S York Street. Building C, Denver,
Co, U.S. 80210
Technologies: Fuel Cell
A fuel cell is a device that generates electricity through a chemical reaction between the cathode and anode. Every fuel
cell has two electrodes, one positive and one negative, called, respectively, the anode and cathode. The reactions that
produce electricity take place at the electrodes. Every fuel cell also has an electrolyte, which carries electrically charged
particles from one electrode to the other, and a catalyst, which speeds the reactions at the electrodes.
Hydrogen is the basic fuel, but fuel cells also require oxygen. One great appeal of fuel cells is that they generate
electricity with very little pollution–much of the hydrogen and oxygen used in generating electricity ultimately combine
to form a harmless byproduct, namely water. Fuel cells can vary from tiny devices producing only a few watts of
electricity, right up to large power plants producing megawatts. Technically, Fuel cells are produced in different types of
proton exchange membrane fuel cell (PEMFC), direct methanol fuel cell (DMFC), Solid oxide fuel cells (SOFC), Alkaline
fuel cells (AFC), Molten carbonate fuel cells (MCFC) and Phosphoric acid fuel cells (PAFC).

We try to create a culture that allows the people to
integrate sustainability into every part of our day-to-day
operations. RSGC team peruses....
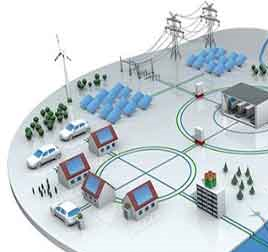
Smart Grid
Smart Grid is the gateway to relibale and
efficient electric grids which supports the
developement of the smart generation,
smart metering and smart control. To this
end, the bidirectional communication
between the utility and customers based
on digital technology helps the smart grid
idea. Smart grid consists of controls,
computers, automation, metering and new
equipment to facilitate the accurate and
responsive reaction of the network in both
normal and emergency situations.
In 2015, the United States generated about 4 trillion
kilowatthours of electricity. About 67% of the electricity
generated was from fossil fuels (coal, natural gas, and
petroleum)....
Automation
Renewable Energy
RSGC Policy
14 Dec - 15 Dec
Utility Regulation Conference: Strategies
for Profit and Reliability
The Westin Georgetown, Washington, D.C,
United States of America
11 Jan - 12 Jan
8th Nuclear Power Asia 2017
Melia Hanoi, 44 Ly Thuong Kiet Street,
001235, Hanoi City, Hanoi, Vietnam.
13 Feb - 14 Feb
Floating LNG 2017
London, , United Kingdom
13 Mar - 15 Mar
International Conference on Emerging
Trends In Environmental Engineering
and Pollution Control 2017
Beijing,China